Ángel Alex ander Cabrera
Accurate and interpretable in silico clinical risk assessment for drug-induced liver injury (DILI) from molecular structure
Katherine Titterton, Daniil Boiko, Ángel Alexander Cabrera, Alex Bäuerle, Akshit Tyagi, Shahin Saneinejad, Alex Beatson, Brandon White
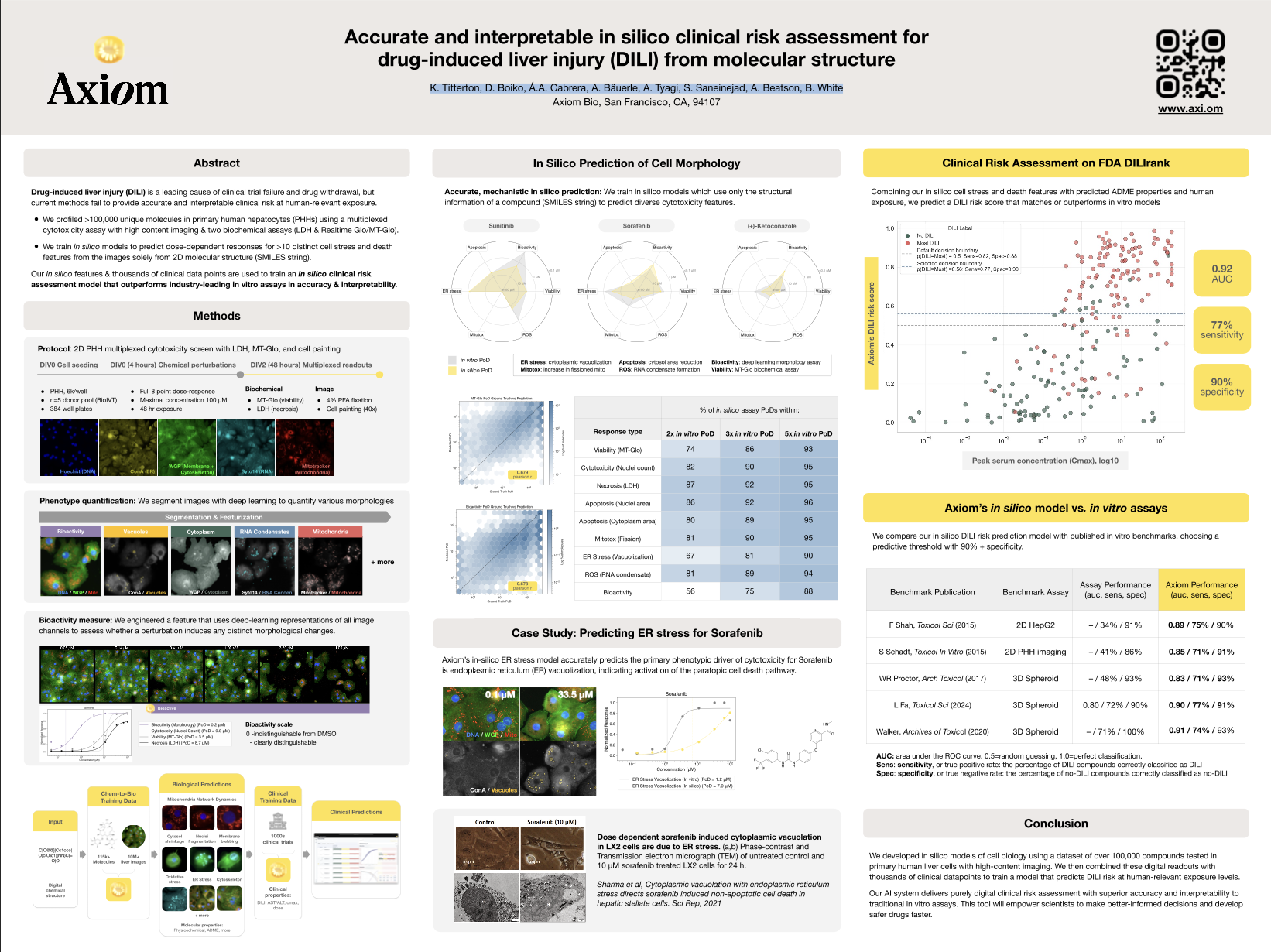
Drug-induced liver injury (DILI) is a leading cause of clinical trial failure and drug withdrawal, but current methods fail to provide accurate and interpretable clinical risk at human-relevant exposure. We profiled >100,000 unique molecules in primary human hepatocytes (PHHs) using a multiplexed cytotoxicity assay with high content imaging & two biochemical assays (LDH & Realtime Glo/MT-Glo). We train in silico models to predict dose-dependent responses for >10 distinct cell stress and death features from the images solely from 2D molecular structure (SMILES string). Our in silico features & thousands of clinical data points are used to train an in silico clinical risk assessment model that outperforms industry-leading in vitro assays in accuracy & interpretability.
Citation
Accurate and interpretable in silico clinical risk assessment for drug-induced liver injury (DILI) from molecular structureKatherine Titterton, Daniil Boiko, Ángel Alexander Cabrera, Alex Bäuerle, Akshit Tyagi, Shahin Saneinejad, Alex Beatson, Brandon White
Society of Toxicology Annual Meeting, Poster. 2025.
BibTex
@article{titterton2025accurate,
title={Accurate and interpretable in silico clinical risk assessment for drug-induced liver injury (DILI) from molecular structure},
author={Titterton, Katherine L and Boiko, Daniil A and Cabrera, {\`A}ngel A and B{\"a}uerle, Alex and Tyagi, Akshit and Saneinejad, Shahin and Beatson, Alex and White, Brandon},
year={2025},
}